Video Description Title
Video Description description
The information contained in the site is intended for US healthcare professionals only. By selecting “Okay” below, you verify that you are a licensed AU healthcare professional.
NoClaudins are present throughout the body, but 2 specific isoforms of CLDN18 are localised to certain tissue types5,6
CLDN18.1
CLDN18.1 is the dominant isoform in normal and malignant lung tissue.
CLDN18.2
CLDN18.2 is the dominant isoform in normal gastric tissue and is often retained in malignant transformation.
Matteo Fassan, MD, PhD
CONFINED IN HEALTHY TISSUE
RETAINED AND EXPOSED IN MALIGNANT TRANSFORMATION
MAINTAINED IN METASTATIC PROGRESSION
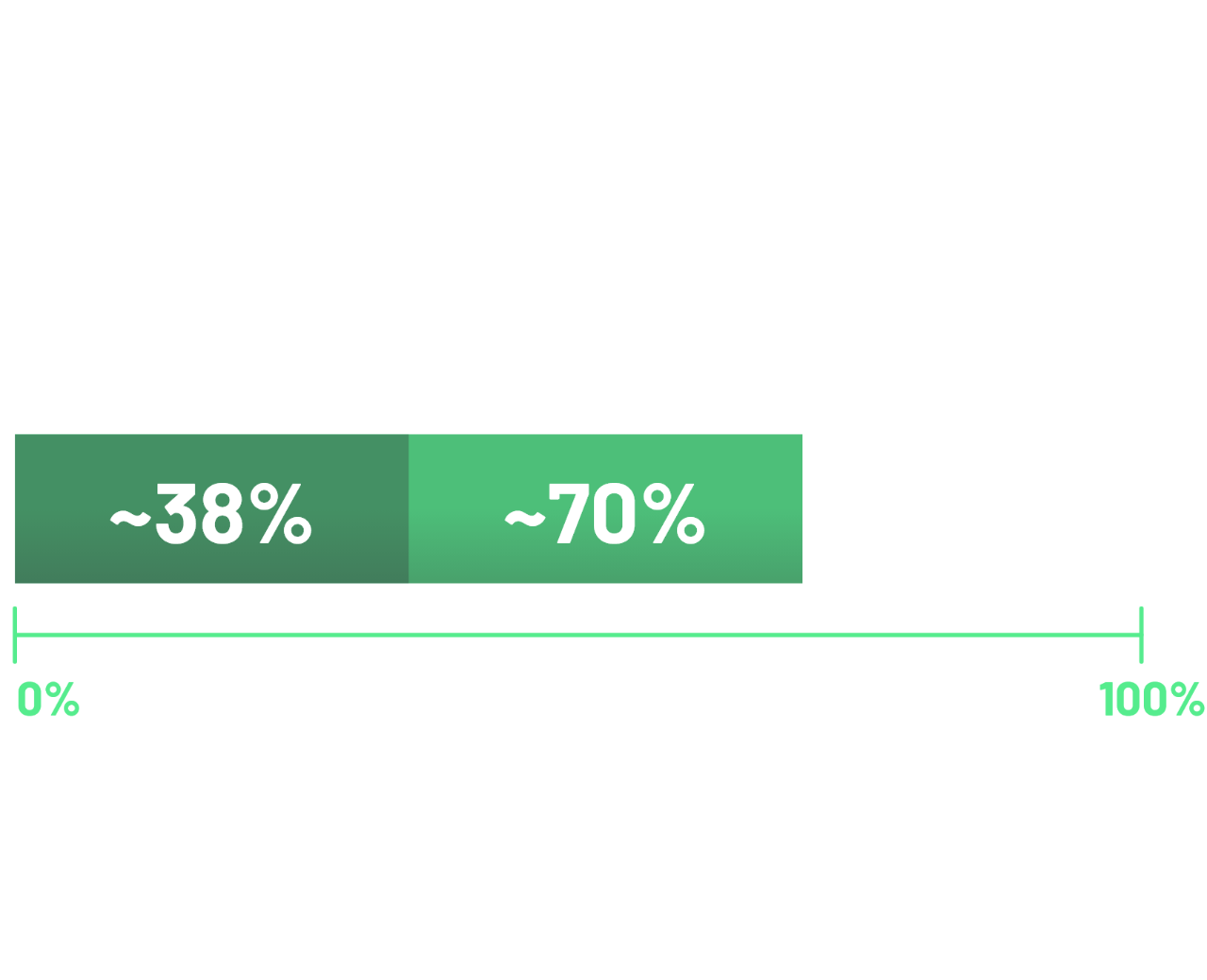
According to 2 recent global studies in patients with locally advanced unresectable or metastatic G/GOJ adenocarcinoma, ~38% of cases demonstrated ≥75% of tumour cells with moderate-to-strong (2+/3+) membranous CLDN18 staining.11,12
CLDN18.1, claudin 18 isoform 1; CLDN18.2, claudin 18 isoform 2; dMMR, deficient mismatch repair; ESMO, European Society for Medical Oncology; GCs, gastric cancers; GOCs, gastro-oesophageal cancers; G/GOJ, gastric/gastro-oesophageal junction; HER2, human epidermal growth factor receptor-2; IHC, immunohistochemistry; PD-L1, programmed death ligand 1; TNM, tumour node metastases.

*Times varied between collection of archival and baseline samples (21 to 1306 days)
RESECTION OF GASTRIC CANCER
Data in patients with G/GOJ cancers suggest that CLDN18.2 expression demonstrated high concordance between primary and metastatic tumour samples.9
In a study of 523 primary G/GOJ adenocarcinomas and 135 pair-matched, synchronous modal metastases9:
As is the case with other biomarkers such as HER2, CLDN18.2 expression may demonstrate variability within a tumour, and this should be taken into account when sampling.9,19
In the same study that demonstrated high-level concordance between primary and metastatic samples, intratumoural heterogeneity in terms of CLDN18.2 expression was found in9:
of primary GC tumours
of primary GOC tumours
of nodal metastases

Stay up to date
By signing up below, you will receive updates about testing for CLDN18.2 expression. You may also be contacted by an Astellas representative. Sorry we could not do signup for your emailID due to technical issue, try after sometime or connect with administrator for more help
Thank you. Your submission was successful.
References: 1. Pellino A, Brignola S, Riello E, et al. Association of CLDN18 protein expression with clinicopathological features and prognosis in advanced gastric and gastroesophageal junction adenocarcinomas. J Pers Med 2021;11(11):1095. 2. Tsukita S, Tanaka H, Tamura A. The claudins: from tight junctions to biological systems. Trends Biochem Sci 2019;44(2):141-52. 3. Hu YJ, Wang YD, Tan FQ, Yang WX. Regulation of paracellular permeability: factors and mechanisms. Mol Biol Rep 2013;40:6123-42. 4. ESMO Gastric Cancer Living Guidelines (07-2023). https://www.esmo.org/living-guidelines/esmo-gastric-cancer-living-guideline/diagnosis-pathology-and-molecular-biology/article/diagnosis-pathology-and-molecularbiology. Accessed 09-07-2023. 5. Sahin U, Koslowski M, Dhaene K, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res 2008;14(23):7624-34. 6. Niimi T, Nagashima K, Ward JM, et al. Claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol 2001;21(21):7380-90. 7. Sahin U, Schuler M, Richly H, et al. A phase I dose-escalation study of IMAB362 (Zolbetuximab) in patients with advanced gastric and gastro-oesophageal junction cancer. Eur J Cancer 2018;100:17-26. 8. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelialmesenchymal transition. Nat Rev Mol Cell Biol 2014;15(3):178–96. 9. Coati I, Lotz G, Fanelli GN, et al. Claudin-18 expression in oesophagogastric adenocarcinomas: a tissue microarray study of 523 molecularly profiled cases. Br J Cancer 2019;121(3):257-63. 10. Rohde C, Yamaguchi R, Mukhina S, Sahin U, Itoh K, Türeci O. Comparison of claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol 2019;49(9):870-6. 11. Shitara K, Lordick F, Bang YJ, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet 2023;401(10389):1655-68. 12. Xu RH, Shitara K, Ajani JA, et al. Zolbetuximab + CAPOX in 1L Claudin-18.2+ (CLDN18.2+)/HER2– locally advanced (LA) or metastatic gastric or gastroesophageal junction (mG/GOJ) adenocarcinoma: primary phase 3 results from GLOW. Presented at: March American Society of Clinical Oncology Plenary Series; March 22, 2023. 13. Van Cutsem E, Bang YJ, Feng-yi F, et al. HER 2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015;18(3):476-84. 14. Fuchs Cs, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated PD-L1- positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer 2022;25:197-206. 15. Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol 2016;22(19):4619-25. 16. Kubota Y, Kawazoe A, Mishima S, et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO Open 2023;8(1):100762. 17. https://www.canceraustralia.gov.au/cancer-types/stomach-cancer/statistics. Accessed 29-08-2024 18. Shitara K, Xu R, Moran D, et al. Presented at the 2023 ASCO Annual Meeting; June 2-6, 2023; Chicago, IL, USA. 19. Grillo F, Fassan M, Sarocchi F, et al. HER2 heterogeneity in gastric/gastroesophageal cancers: from benchside to practice. World J Gastroenterol 2016;22(26):5879-87.